The impact of obesity on mitochondrial function has long been a subject of scientific inquiry. Mitochondria, known as the “powerhouses of the cell,” are crucial organelles responsible for generating energy. However, in individuals with obesity, the functioning of mitochondria is often impaired, leading to a variety of health issues. While the exact reasons behind this impairment are not fully understood, a recent study sheds light on the relationship between obesity and mitochondrial fragmentation.
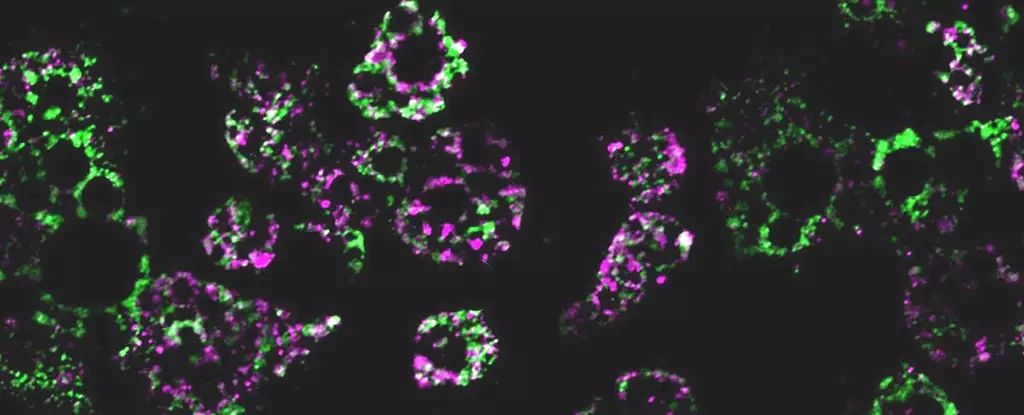
In a groundbreaking research study, an international team of scientists conducted experiments on mice to investigate the impact of a high-fat diet on mitochondria within fat cells. The results revealed that when fed a high-fat diet, mitochondria in the mice’s fat cells broke apart into smaller fragments. These fragmented mitochondria exhibited a reduced capacity for burning fat, which may contribute to weight gain and the further development of obesity-related health complications.
Interestingly, the study also identified a single gene responsible for governing the fragmentation process. When this gene was deleted in the mice, they were able to avoid excessive weight gain, even when consuming the same high-fat diet as their counterparts. The findings highlight the critical role of this gene in transitioning from a healthy weight to obesity. Understanding the mechanisms behind this transition is crucial for developing targeted therapies to address weight gain and associated metabolic dysfunctions.
Obesity has become a significant public health crisis worldwide, with rates nearly tripling over the past 50 years. This surge in obesity prevalence brings with it a range of serious health complications, including diabetes, heart disease, and cancer. With excess fat accumulation in adipose tissue as a defining characteristic of obesity, understanding the underlying metabolic anomalies is of utmost importance.
The Complex Functions of Adipose Tissue
Adipose tissue, which consists of fat cells, contributes to various mechanical and metabolic processes in the body. These include cushioning organs and releasing cellular signaling molecules. However, in individuals with obesity, fat cells can become less efficient at burning energy, making weight loss even more challenging. The origins of this metabolic anomaly remain elusive, prompting researchers to delve deeper into understanding the complexities of obesity.
The study discovered that the fragmentation of mitochondria in fat cells, induced by a high-fat diet, is controlled by a molecule called RalA. RalA is a versatile molecule with multiple functions, including the breakdown of malfunctioning mitochondria. However, an overactive RalA could potentially disrupt the normal functioning of mitochondria, leading to a cascade of metabolic dysfunctions associated with obesity.
Implications for Targeted Therapies
By elucidating the mechanism involving RalA, the researchers have brought us one step closer to developing targeted therapies for weight gain and associated metabolic dysfunctions. In a fascinating experiment, the team deleted the gene associated with RalA in some mice while subjecting them to the same high-fat diet as the control group. The mice without the gene were able to avoid diet-induced weight gain, highlighting the potential of targeted interventions in treating or preventing obesity.
Translating Findings to Human Health
Although this study was conducted on mice, the researchers noted striking similarities between certain RaIA-influenced proteins in mice and human proteins implicated in obesity and insulin resistance. While further research is necessary to determine the applicability of these findings to humans, the correlation between fundamental biological mechanisms and clinical outcomes emphasizes the potential for developing new therapies targeting the RalA pathway.
The groundbreaking study sheds light on the intricate relationship between obesity and mitochondria fragmentation. Understanding the mechanisms underlying this connection has the potential to revolutionize the treatment and prevention of obesity and its associated health complications. By targeting the RalA pathway, scientists may pave the way for more effective therapies that promote fat burning and mitigate the detrimental effects of obesity on overall health.
Leave a Reply