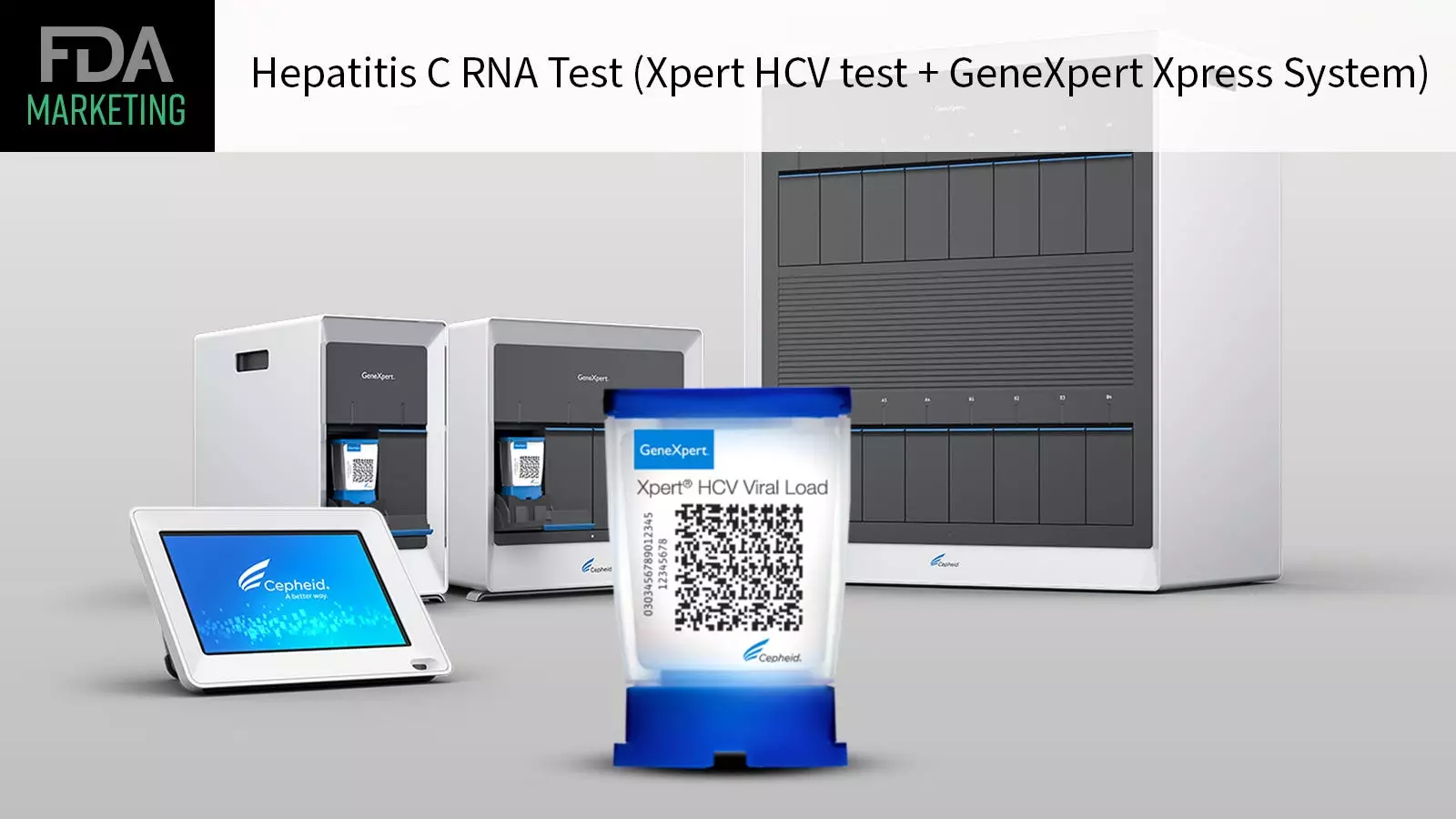
The FDA recently granted marketing authorization to the first point-of-care test for hepatitis C virus (HCV), which allows at-risk adults to be quickly diagnosed and connected to care if necessary. This test, known as the Xpert HCV test and GeneXpert Xpress System, detects HCV RNA and is specifically indicated for individuals exhibiting signs or symptoms of hepatitis C. The convenience of this test lies in the fact that it can be used at various healthcare settings, such as doctors’ offices, urgent care centers, and emergency rooms, without the need to send samples to a central lab.
The Xpert HCV test can deliver results within an hour using a simple fingertip blood sample, enabling healthcare providers to promptly diagnose patients and discuss treatment options during the same visit. This immediate link to care can prevent disease progression and further transmission of the virus. The director of the FDA’s Center for Devices and Radiological Health, Jeff Shuren, emphasizes the importance of widespread testing options for hepatitis C, as many individuals are unaware of their infection status due to limited testing availability.
Although effective direct-acting antivirals for HCV have been available for years, the cure rates in the U.S. remain low. The CDC recommends screening all adults for HCV at some point in their lives, as early detection is crucial in preventing severe complications such as liver cancer or liver failure. Unfortunately, the current testing process often involves multiple appointments for testing, results, and follow-up, leading to some infected individuals slipping through the cracks and not receiving appropriate care.
Hepatitis C affects an estimated 4 million people in the U.S. and was associated with over 12,000 deaths in 2022. Many individuals infected with HCV are asymptomatic or experience mild symptoms, making it challenging to diagnose without routine screening. Jaundice, poor appetite, nausea, and fatigue are common signs of infection, indicating the need for improved testing methods to reach undiagnosed individuals.
The approval of the Xpert HCV test marks a significant advancement in hepatitis C diagnosis and treatment. Dr. Jonathan Mermin, director of CDC’s National Center for HIV, Viral Hepatitis, STD, and TB Prevention, highlights the importance of making this test affordable and readily available to maximize its impact on curing hepatitis C infections. However, it is essential to note that the test is not intended for monitoring patients undergoing HCV treatment or for screening blood or tissue donors.
Like any medical test, the Xpert HCV test carries risks of false-positive and false-negative results, which can lead to unnecessary fears, treatment delays, or other consequences. It is crucial for healthcare providers to interpret the test results accurately and follow up with confirmatory testing if needed to ensure the correct diagnosis and treatment plan for patients.
The FDA’s approval of the first point-of-care test for hepatitis C virus is a significant step towards improving the diagnosis and treatment of this infectious disease. By offering a rapid and convenient testing option, more individuals can be identified and linked to care, ultimately reducing the burden of hepatitis C on public health. Continued efforts to increase access to testing and treatment will be essential in eradicating hepatitis C and preventing future complications associated with this virus.
Leave a Reply