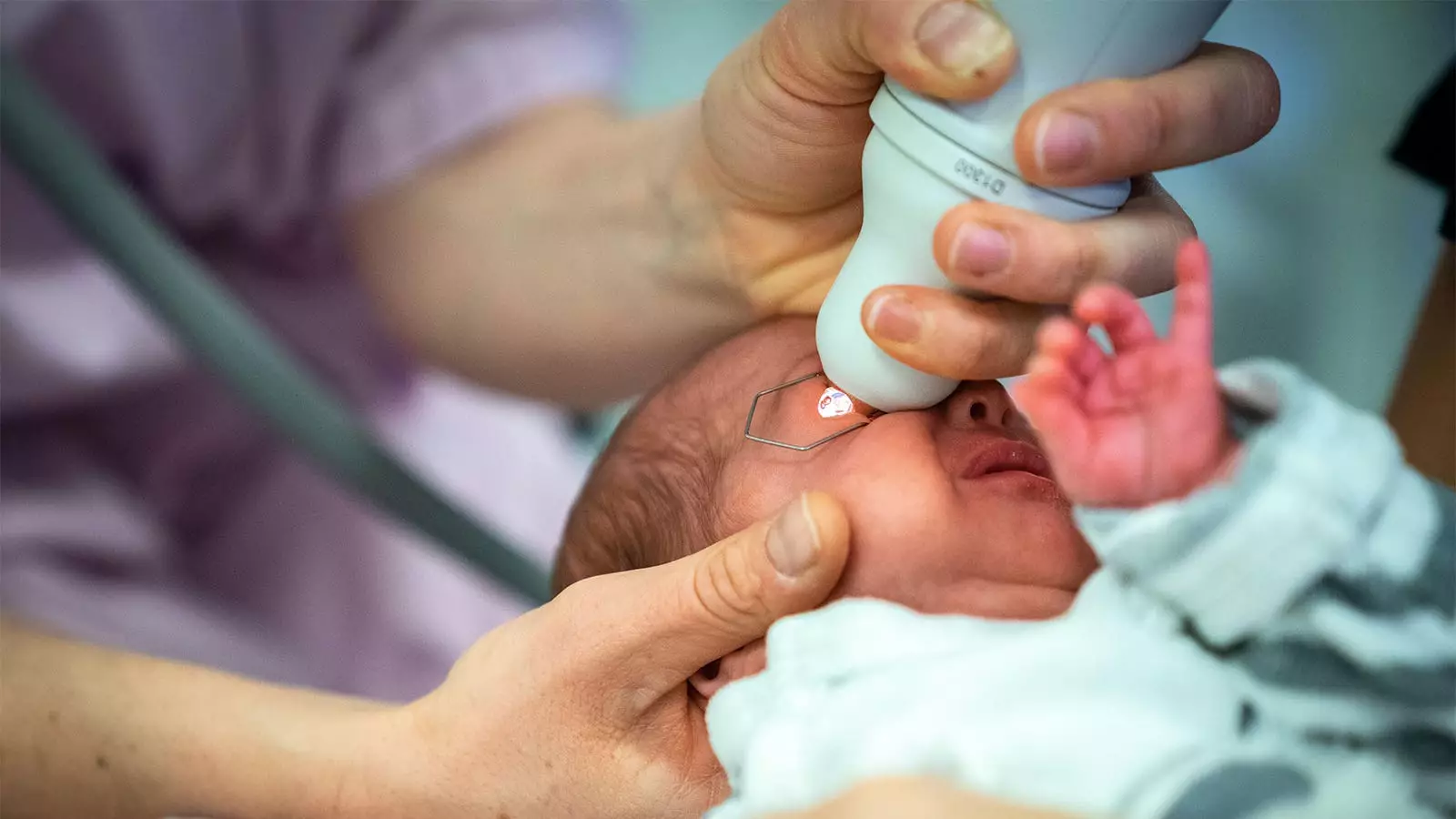
Retinopathy of Prematurity (ROP) is a significant concern in neonatology, affecting premature infants, particularly those born before 32 weeks of gestation. The condition can lead to severe vision impairment or blindness if not promptly and effectively treated. Regular eye examinations for ROP necessitate the dilation of pupils, which is typically achieved with the use of mydriatic eyedrops. However, standard formulations have been linked to a variety of systemic adverse events that raise concerns among healthcare professionals. With the growing risks associated with these traditional drops, researchers have been exploring safer alternatives, paving the way toward improved ocular care for at-risk infants.
Recent research spearheaded by Asimina Mataftsi, MD, PhD, and her colleagues at Aristotle University of Thessaloniki, has demonstrated that mydriatic microdrops present a promising alternative to standard dilation drops. In a randomized trial involving 83 preterm infants, microdrops were not only found to be noninferior to standard drops but also excelled in mydriatic efficacy—particularly at the critical 45-minute mark. This study posits microdrops as a viable solution to counterbalance the constraints posed by traditional methodologies in ROP screening, especially for the fragile physiology of preterm infants.
The trial revealed that microdrops led to significant mydriatic effects compared to standard drops, with a remarkable mean difference of 0.12 mm in pupil diameter at 45 minutes post-instillation, subsequently showing noninferiority at 90 and 120 minutes. The findings are indicative of the potential for microdrops to enhance the efficacy of ROP screening while minimizing the associated risks.
One of the key advantages presented by mydriatic microdrops is their reduced risk of systemic side effects, particularly concerning cardiorespiratory and gastrointestinal complications. In the study, the use of standard drops was correlated with lower oxygen saturation levels at 45 and 90 minutes post-administration, which is critical in this vulnerable population. The results indicated an increase in hypertensive episodes among those treated with standard drops, amplifying the argument for adopting microdrops in clinical practice.
Mataftsi and her team argued that these findings underscore a growing body of evidence suggesting that standard mydriatic treatments can exacerbate pre-existing medical conditions among premature infants. The reduced volume in microdrop administration points to a promising future in minimizing medication-related harms while maintaining effective dilation.
The importance of continuous research in neonatal medicine cannot be overstated. Given the intricacy of care for premature infants and their susceptibility to adverse reactions from medications, studies like the MyMiROPS trajectory are vital. They not only affirm existing knowledge but push the boundaries of what can be achieved in clinical settings. The methodology adopted in the study, including careful patient selection based on gestational age and weight, ensures that the findings are both applicable and relevant to a broad spectrum of at-risk infants.
Moreover, the study highlights essential gaps in current ROP treatment protocols, prompting the medical community to move toward more refined and patient-tailored approaches. The researchers acknowledged limitations, including safety data gaps for a portion of participants, thus emphasizing the need for further studies to validate and expand upon their findings.
The recent clinical trials affirming the efficacy of mydriatic microdrops present exciting possibilities for the future of ROP screening in premature infants. As the medical community grapples with the challenges of safeguarding one of its most vulnerable patient populations, research initiatives such as those by Mataftsi et al. underscore the importance of innovation in therapeutic strategies. Moving forward, a pragmatic and evidence-based approach must be promoted, balancing efficacy with safety in the delicate task of caring for premature infants who are at risk of ROP. This new frontier in mydriatic solutions not only marks a step forward in eye care but also reinforces the overarching goal of protecting the health of our youngest patients.
Leave a Reply