In a recent decision, the Food and Drug Administration (FDA) approved Moderna’s vaccine for respiratory syncytial virus (RSV) for adults ages 60 and above. This approval marks a significant milestone for Moderna, as it is the company’s second-ever product to enter the U.S. market. The approval comes at a crucial time for Moderna, as demand for its Covid jab, its only commercially available product, has been declining. The decision to approve Moderna’s shot was based on a late-stage trial on older adults, who are more susceptible to severe cases of RSV. This virus annually causes between 6,000 and 10,000 deaths in seniors and leads to 60,000 to 160,000 hospitalizations, according to data from the Centers for Disease Control and Prevention.
Market Impact
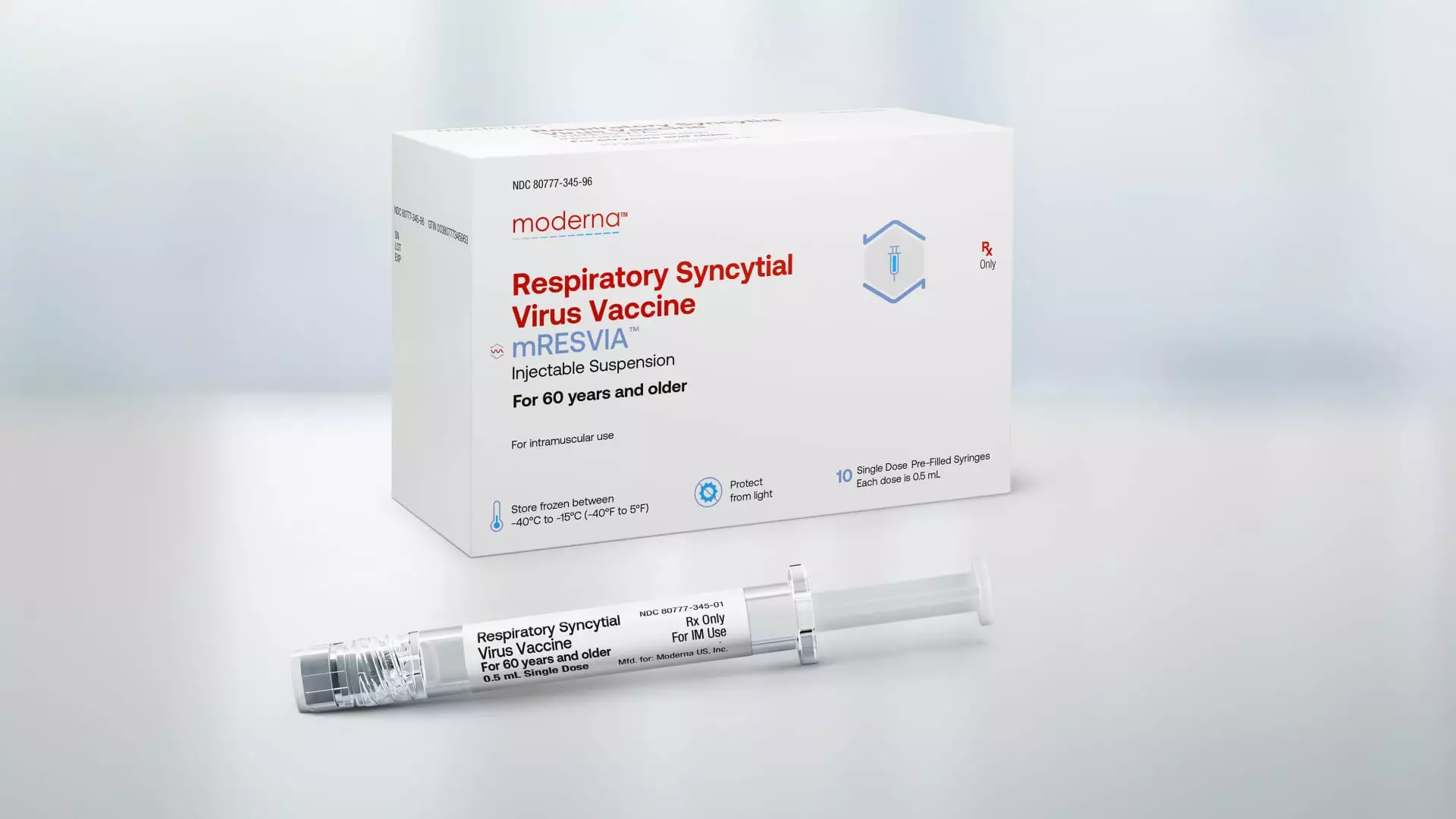
Moderna’s vaccine for RSV will be marketed under the brand name mRESVIA. It is the first messenger RNA vaccine to receive approval for a disease other than Covid. Additionally, Moderna’s shot is the only RSV vaccine available in a pre-filled syringe, making it easier for healthcare providers to administer to patients. An advisory panel to the CDC is set to vote in June on recommendations for the use and intended population for Moderna’s shot. Moderna expects a favorable recommendation, which would allow its vaccine to compete with existing RSV shots from pharmaceutical giants GSK and Pfizer, both of which launched their respective shots in the U.S. last fall.
Revenue Forecast
Moderna’s full-year 2024 sales guidance includes revenue from its RSV vaccine, projecting roughly $4 billion in sales. This approval demonstrates the versatility of Moderna’s messenger RNA platform beyond treating Covid. The company is leveraging this technology to address a range of diseases, such as RSV, cancer, and norovirus, a highly contagious stomach bug. Moderna’s CEO, Stéphane Bancel, emphasized the potential impact of mRESVIA in addressing global public health threats related to infectious diseases.
Modern currently has over 40 products in development, including a combination shot targeting both Covid and the flu, expected to win approval by 2025. The company is also working on a stand-alone flu shot, a personalized cancer vaccine in collaboration with Merck, and shots for latent viruses. Moderna’s long-term forecast anticipates a return to sales growth in 2025 and aims to break even by 2026 through the launch of these new products. Investors are optimistic about the potential of Moderna’s mRNA product pipeline, as evidenced by the company’s stock performance, which has increased by over 40% this year.
During the FDA’s review process, Moderna faced some concerns regarding the efficacy of its RSV vaccine. A phase three trial involving approximately 37,000 participants demonstrated that Moderna’s vaccine was 83.7% effective at preventing at least two symptoms of RSV at three months. However, new data in February indicated a decline in efficacy to 63% at 8.6 months. This led to speculation among investors that Moderna’s vaccine might not perform as well as those from GSK and Pfizer. Moderna countered these concerns by highlighting the differences in study populations, geographic locations, and case definitions for RSV, emphasizing the need for head-to-head trials to draw accurate comparisons.
The approval of Moderna’s vaccine for RSV in older adults represents a significant achievement for the company. With a robust product pipeline and a commitment to leveraging mRNA technology for a range of diseases, Moderna is poised for continued growth and innovation in the healthcare industry.
Leave a Reply