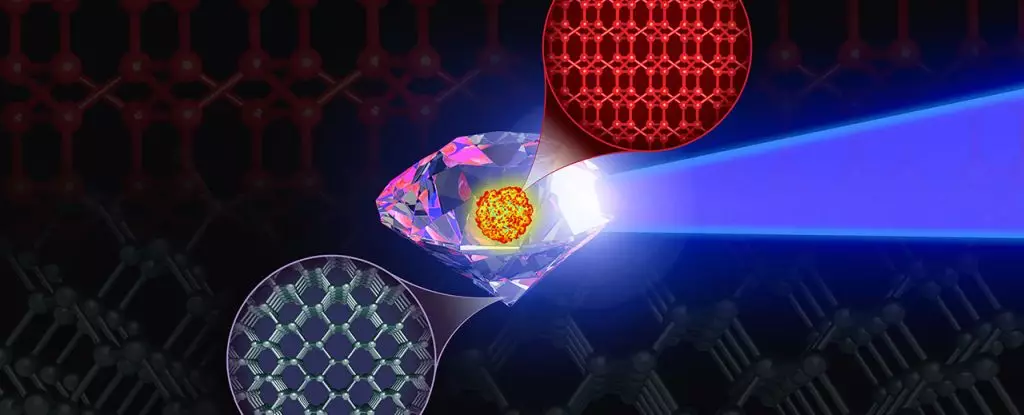
A recent study conducted by physicists from the US and Sweden has shed light on the elusive eight-atom body-centered cubic (BC8) phase of carbon. This phase is believed to be up to 30 percent more resistant to compression than diamond, which is currently the hardest known stable material on Earth. The researchers utilized quantum-accurate molecular dynamics simulations on a supercomputer to investigate how diamond behaves under high pressure and temperature conditions that would typically make it unstable.
The BC8 phase has previously been observed in silicon and germanium on Earth, providing scientists with insights into how this unique structure could manifest in carbon. While it is not naturally found on Earth, it is believed to exist in the high-pressure environments deep within exoplanets. Theoretical calculations suggest that BC8 carbon could be the hardest form of the element that remains stable at pressures exceeding 10 million times Earth’s atmospheric pressure.
Despite the potential benefits of harnessing the BC8 phase for various applications, attempts to synthesize it in a laboratory have been unsuccessful. Researchers, led by physicist Kien Nguyen Cong, turned to the power of supercomputing to unravel the mysteries surrounding the formation of BC8 carbon. By running simulations on the world’s fastest supercomputer, Frontier at Oak Ridge National Laboratory, they were able to uncover the key obstacles hindering the synthesis of this elusive molecule.
The team’s simulations revealed that the post-diamond BC8 phase of carbon can only be achieved under very specific high-pressure and high-temperature conditions. This narrow region in the carbon phase diagram has eluded experimental synthesis attempts thus far. However, armed with this new knowledge, scientists are hopeful that targeted efforts can finally yield success in creating BC8 carbon in a laboratory setting.
The discovery of the BC8 phase opens up a realm of possibilities for materials science and research. With its potential for enhanced hardness and stability, BC8 carbon could revolutionize various industries, from electronics to aerospace. Understanding the conditions required for its formation brings us one step closer to unlocking the full potential of this elusive carbon molecule.
The quest for BC8 carbon represents a significant scientific breakthrough with far-reaching implications. Through the power of supercomputing simulations, researchers have unraveled the mysteries surrounding this elusive molecule, paving the way for new discoveries and applications in the field of materials science. The journey to synthesizing BC8 carbon may have been challenging, but with perseverance and innovation, the scientific community is well on its way to unlocking the secrets of this remarkable material.
Leave a Reply